The Next One
Preparing Canada for Another Health Emergency
Introduction
COVID-19 has killed over 53,000 Canadians, more than the Second World War and the Korean and Afghanistan wars combined.[1] Since there is no such thing in public health as a ceasefire or peace treaty, those charged with defending the country’s health security can never again let their guard down.
And that raises an alarming question: Are we winning the peace?
COVID-19 descended on us in early 2020 with deadly power. It spread easily and rapidly worldwide, proliferating before many governments and public health officials fully understood the breadth of the threat it posed. It forced extreme measures such as social distancing and sheltering in place. Ultimately, vaccines that were developed, tested and distributed in record time turned the tide. Science, and life sciences in particular, played the role of saviour.[2]
The next pandemic or the next health threat may well defy our expectations again. The only thing we know for sure is there will continue to be major health threats in the future. In what form, where and when are all unknowns, which means we must be prepared for a public health enemy that we cannot truly identify.
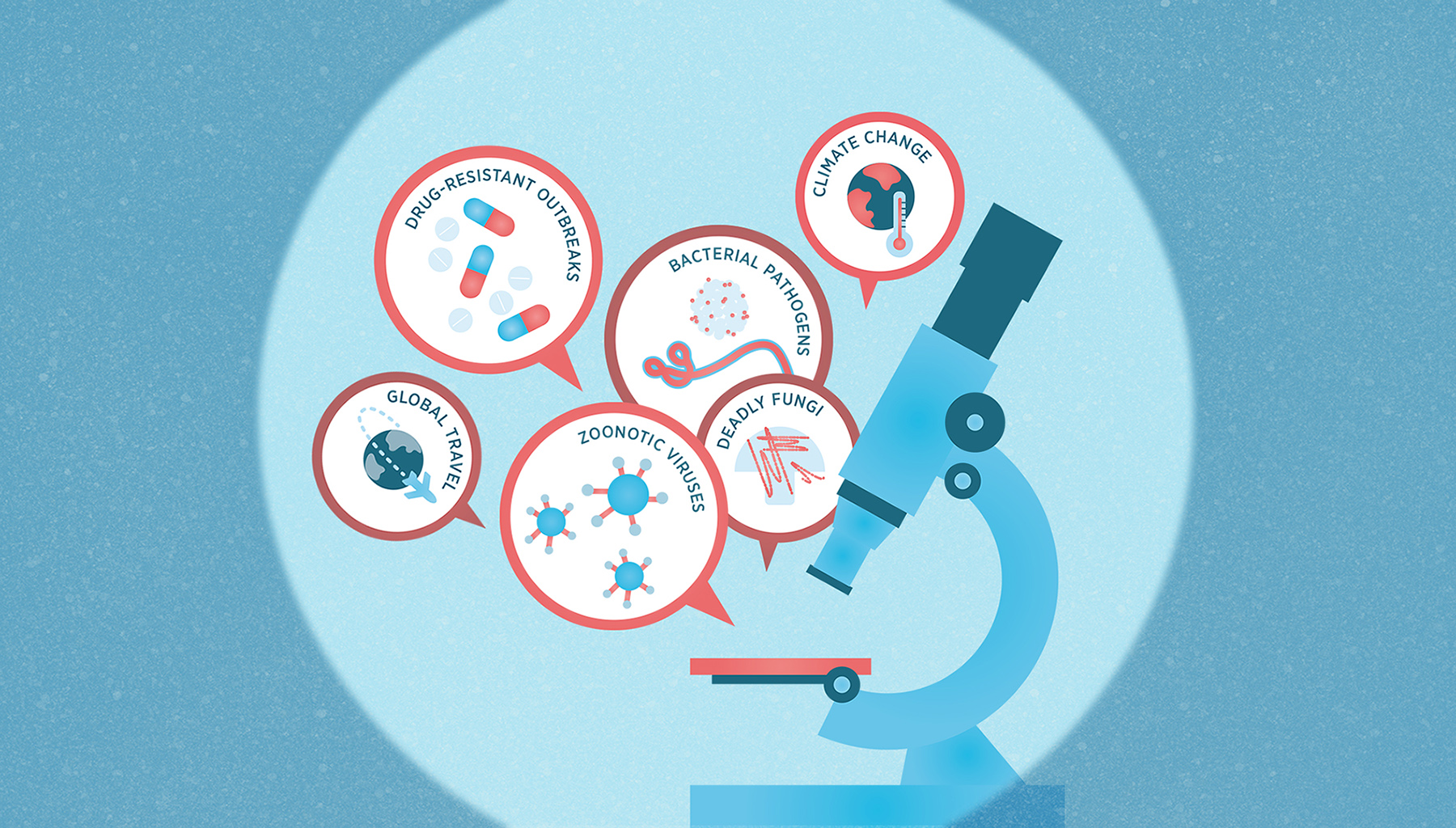
Humans are adaptable. We normalize omnipresent threats and focus on short-term problems rather than long-term ones. As the immediate threat posed by the pandemic recedes, it’s natural that our attention is diverted to the problems we face today. Yet the probability of another pandemic occurring within one’s lifetime is roughly 38 percent and may even grow to an extraordinary 76 percent within the next few decades.[3] Trends such as globalization, inter-regional travel, urbanization and climate change are fueling the increased incidence of disease outbreaks.
Before COVID, Canada had the luxury of not thinking of health in terms of public safety. Today, the term health security has entered the lexicon. According to intelligence gathered by the World Health Organization, respiratory pathogens such as COVID and influenza will likely be the cause of a future pandemic due to their high mutation rates and transmissibility. But health security is equally jeopardized by the growing presence of diseases associated with environmental change that have epidemic potential, such as Candida auris (C. auris), a deadly fungus that affects those with weak immune systems[4] and increases the probability of extreme epidemics. Zoonotic diseases — those that originate in animals and spread to humans, such as avian flu — are of particular concern as a source of pandemics due to their unpredictability and potential for rapid spread.[5]
Canadians’ health security could also be undermined by an emergency triggered by our changing climate. Canada has seen a record number of forest fires in 2023[6] — by September, an astonishing 15-million-plus hectares had already been burned, equal to the combined land area of Nova Scotia and New Brunswick. This summer also brought historic levels of flooding and heat, tornados and choking atmospheric pollution across the country. The coming winter could well mean more ice storms and floods. In short, climate change is already causing excess deaths, the paralysis or isolation of communities, and the destruction of infrastructure needed for human survival.
As the intensity of COVID recedes and before the next public health threat materializes, this is the moment to prepare defences to protect our collective health security. It requires, as in the teeth of the pandemic, bringing together different, sometimes antagonistic, parties in the life sciences sector by building peacetime trust, partnerships and a sense of common purpose among industry, governments, academics and research institutes.
A true health security approach means taking measures to mitigate threats before they emerge, using the period of calm to prepare for the storm, just as infrastructure must be built not for the sunny days but for the once-in-100-years storms.
Investments must be made today, building on our existing strengths as well as the lessons learned from the pandemic. Such investments might look redundant — until suddenly one day they are not. We need to expand intelligence-gathering systems, while further enhancing domestic capacities and co-ordinating policies and systems. Most of all, we need to make Canada an indispensable player in the global life sciences ecosystem and its supply chains.
Last time out, Canada had little to trade and needed to pay dearly to secure vaccines. With just two percent of the global market and scant vaccine manufacturing capabilities or other assets, we were forced to spend more and buy more than needed to get suppliers to push their vaccine pallets toward a Canadian plane. For its part, Israel secured some of the earliest vaccine shipments by offering itself as a “pilot country” with access to world-leading health data systems, including insights into adverse reactions.[7]
As global supply chains tighten and other nations bolster their capabilities and attractiveness as buyers, the imperative becomes ever more urgent. Canada needs to use this interregnum to identify and develop areas of comparative advantage that we can turn into strategic tradeables — our version of Israel’s health data. As Kate Bingham, the COVID-era British vaccine czar, wrote in her book The Long Shot: The Inside Story of the Race to Vaccinate Britain, smaller markets must identify their strengths and play to these to increase their relevance vis-à-vis the purchasing power of the United States, European Union or Japan. “We decided that the only way we could compete with these massive buyers was by turning the U.K. into the best possible client.”[8]
Success next time begins with building trust today. In the desperation of the pandemic, industry, government and others in Canada were able to overcome long-held suspicions in favour of co-operation. In the aftermath, relationships cannot be permitted to retreat.
To help achieve the goals of better preparedness and heightened health security, the Public Policy Forum has established a Life Sciences Forum — a regular meeting place where organizations come together under a shared goal of bolstering the country’s health security. The Life Sciences Forum engages those from industries, such as pharmaceuticals and medical technologies, as well as federal and provincial governments and regulators, academics, non-profits, civil society leaders and researchers. Canada’s health security requires a coalition pulling in the same direction.
The cross-sectoral, pan-Canadian and co-operative nature of our Life Sciences Forum is unique in this country and, anecdotally, also extraordinarily rare among Canada’s peers. Its goal is to help foster the collaborations and advance strategies that will better prepare Canada to respond quickly to the next health threat.
Similarly, it is PPF’s goal to extend this work to considerations bearing on the broader health security of Canadians. We win the next war by first winning the peace.
The context for action
Although the term “health security” has a range of definitions, the Life Sciences Forum is guided by the version offered by the World Health Organization: “the activities required, both proactive and reactive, to minimize the danger and impact of acute public health events that endanger people’s health across geographical regions and international boundaries.”[9]
Notably, there is both a proactive and reactive component to this definition. To have a high level of health security, a country must proactively invest in a strong life sciences sector, with a baseline set of capabilities to draw upon and ramp up during a crisis. Health security is multi-dimensional, including risk assessment, emergency planning and management, communications among players and with the public, contributions to and procurement from global product supply chains, as well as the flexible, crisis-driven surge capacity of those supply chains and the country’s health-care systems.
Canada’s life sciences sector mobilized and responded to COVID with unprecedented speed. Past suspicions and animosities were set aside, traditional health-care delivery hierarchies went out the window, and businesses mobilized to, in some cases, master and manufacture products they had never produced. Distrust in the sector, rooted in debates around pricing and market access, took a back seat to providing the research, equipment and pharmacological products needed to minimize COVID infections and secure the health and safety of Canadians.
Much of it was an unprecedented success, demonstrating how, by working together, governments and the life sciences sector can build the public trust essential to implement a speedy and effective response to a pandemic. Canadians reacted positively, with 87 percent of those aged five years and older receiving at least one dose of the COVID vaccine and 84 percent completing the primary series of vaccine doses.[10]
The COVID experience also highlighted how a vibrant life sciences sector can play a critical role in Canada’s future growth and competitiveness. The federal and provincial governments and the life sciences sector are building on recent initiatives, such as filling the manufacturing void and allocating $2.2 billion in the 2022 federal budget to a national Biomanufacturing and Life Sciences Strategy.[11] Provinces are also focused on supporting their life sciences sectors, promoting more research and innovation across the country. There is specific focus on areas where we have potential global comparative advantages, such as the utilization of artificial intelligence for drug discovery and the breadth and diversity of data emerging from Canada’s clinical trials. Also critical will be a level of fundamental research to prepare for potential threats, as well as capability to scale up and ultimately distribute diagnostics, vaccines and therapeutics to counter health threats.
The pandemic also revealed glaring shortcomings in Canada’s health security emergency preparedness. For example, the auditor general of Canada recommended that barriers be resolved to better share information on vaccine surveillance and adverse effects among Canadian government agencies, and that such data also be quickly shared with vaccine companies and the World Health Organization.[12] The auditor general also recommended the Public Health Agency improve “its automated tracking and data quality” to better follow up with travelers, and that it undertake gender-based analysis to address potential adverse effects of programs on diverse and vulnerable groups.[13]
Recommendations for immediate action
Canada has a precious window of opportunity — right now, today — to draw lessons from the pandemic and apply them to urgent readiness planning. But the window is closing.
For instance, the heads of Canadian subsidiaries of many multinationals involved in COVID vaccine research and production have changed jobs, taking with them the memory of collaborative dialogue, risk-taking and co-ordinated focus that defined Canada’s collective COVID response. As the system settles back into regular operations and other important policy priorities take centre stage, the impetus for action experienced during the pandemic is fading. As well, many Canadians are eager to put the experience of lockdowns, masking and other restrictions — with its many societal and economic consequences — behind them.
But not just yet. The country’s future health security is threatened by such slippage.
Canadians will not be forgiving — nor should they be — if the country is caught ill-prepared in the face of the next health threat. Canadians will demand more next time, and better. Decision-makers can’t set the past aside until they have strengthened the country’s health security and preparedness for the next emergency. This window cannot be permitted to close while planning remains undone. Not yet.
These recommendations flow from information gathered by the Life Sciences Forum to date and outline the actions Canada must take to prepare for the next health emergency, as well as suggesting improvements to our response when that emergency occurs.
They also reflect the logical progression in policy development that begins with determining the strategy to increase preparedness. That is followed by designing a structure and organization specifically to shape and oversee the implementation of the processes and activities that will lead to the desired result — greater health security for Canadians.
Strategic management and direction to build Canada’s comparative advantage
Recommendation 1.1 – Create an institution to oversee health security.
The pandemic laid bare a glaring absence: Canada lacks an institution to connect a chain of urgent requirements and roles in the face of a health crisis. Goodwill among Canada’s allies, quick approval of vaccines and test kits, willingness to pay for procurement and the creation of new task forces and science tables — notably with clear mandates to provide advice — got the job done against all odds.
Through this experience, we have learned that clear governance and authority must be created to enable planning, testing and preparedness. Other countries are applying this lesson and creating institutions such as the U.S. Biomedical Advanced Research and Development Authority (BARDA), the European Union’s Health Emergency Preparedness and Response Authority (HERA), and the United Kingdom’s Advanced Research and Invention Agency (ARIA). All have clear mandates from national governments to ensure their countries’ health security, and the authority to make investment and procurement decisions that enable this mission.
What should a new entity look like in Canada?
It should be an institution charged with preparing for health emergencies by bringing together partners from industry and government with the authority to act quickly, as well as making investments to bolster Canada’s health security both between and during crises.
A “Canadian BARDA,” fueled by reliable and immediately available federal funding, must be given a mandate to detect and respond to health emergencies, ensuring diagnostics, medicines, vaccines and other medical countermeasures are rapidly developed, manufactured or acquired through innovative procurement practices. It should focus on assessing threats, supporting research and innovation, addressing market changes and considering industrial capacity and global supply chains. However, it must also engage with and acknowledge the central role of provinces and territories in running health systems, distributing drugs, therapeutics and medical devices, and making purchasing decisions.
As BARDA does in the United States through its five-year plans, the new entity must undertake regular updates to Canada’s health security strategic plan, including public reporting on the status of the health security system.
To be effective, this entity also needs to be staffed with top, internationally connected talent drawn from around the world. Within Canada, it must bring together partners from across the life sciences value chain to build systems that ensure the ability to produce or procure whatever is deemed necessary, including procurement from multiple suppliers, as occurred with COVID vaccines. The unwavering goal: to address future health threats, even before the extent and impact of a specific threat is known.
Strategic questions:
Implementing each of these recommendations involves responding to a series of strategic questions, as well as identifying clear targets and objectives. Specific concerns and questions flagged by the Life Sciences Forum include:
- What lessons can Canada apply from the COVID experiences in the U.S., U.K. and EU to shape a new institution to be built to oversee health security?
- What relationship should a “Canadian BARDA” have with its international counterparts and with existing domestic institutions focused on health security and preparedness?
- What essential tasks should be given to this new entity and who and what should it oversee?
- How can we apply lessons learned from emergency response systems used in the COVID-19 crisis to bolster Canada’s response to future health threats? These may include lessons from the use of innovative procurement mechanisms, communications systems, and/or data tracking systems.
- BARDA is currently focusing on advanced research and development, zeroing in on phase one clinical trials for pharmaceuticals coming out of the preclinical development phase. Can Canada establish and support this level of dedication to developing medical preparation and countermeasures in this country?
Recommendation 1.2 – Identify, build and promote niches where Canada can develop a comparative advantage.
While globalization enables a pandemic’s spread, it also helps conquer it; containment is inevitably rooted in global co-operation. No single country had all the ideas, materials, resources, capacity, knowledge base or industrial capability to overcome the pandemic alone. Countries such as Israel, the U.K. and the U.S. leveraged their unique life sciences capabilities at global negotiating tables. Israel used its health data to quickly procure the Pfizer vaccine, the U.K. used its biosciences and bioindustry capabilities, the United States used its market clout — expressed through Operation Warp Speed[14] — to accelerate the development, manufacturing and distribution of vaccines. Like many countries, Canada’s ability to become self-sufficient or compete head-to-head with global life sciences powerhouses is limited by its market size. As such, it’s not about competing, but rather about becoming essential.
Building Canada’s comparative advantage requires the development of strategic tradeables that it can bring to the negotiating table in a crisis — the products, procedures and processes Canada can contribute to the next emergency’s multinational response in exchange for access to essential goods and services required from other nations.[15]
What does the world need that Canada is positioned to provide? How can Canada become essential to global life sciences supply chains? As a small market, we must identify areas and expertise to cultivate and nurture where we can fill a global niche, inserting ourselves into global value and supply chains for health security. In doing so, our emphasis needs to be on preparation rather than reaction, so that our multinational partners know what we can deliver in bulk on short notice when needed.
Canada has strong science. Scientific excellence in stem cell and gene therapies, lipid nanoparticles, etc., means that every COVID vaccine in use has a bit of Canadian research in it. We also have a globally competitive talent pool and, with concerted effort, both assets can provide a comparative advantage. We must:
- Build and exploit the depth of talent that exists in Canada’s life sciences sector, both within its workforce and in the broader academic and research communities;
- Improve the operation of clinical trials to reduce cost while increasing speed and agility in providing results; and
- Develop the capability to build material that the rest of the world needs.
These are the prerequisites for Canada at home and to become a full and valuable partner internationally in responding to future health emergencies.
Strategic questions:
- What capabilities and products can Canada develop to fill gaps in global life sciences value chains?
- Which resources in Canada’s health system are strategic assets that meet best worldwide practices and are tradeable?
- In which fields can Canada become internationally essential, acknowledging that we can’t do everything by ourselves and that the Canadian market is not big enough — nor is domestic access to capital sufficient — to compete directly with major players such as the U.S. or Europe?
- Where does Canada already have, or where can we build, something strategically valuable to offer the world in life sciences?
- Following Israel’s lead of data-for-vaccines, how can Canada leverage its diverse population to produce the most genetically diverse health dataset?
- Canada also has a significant urban-rural divide, with large urban centres and a diverse set of rural communities, including Indigenous communities in remote settings, that provide insight into differing challenges around access, language and equity, as well as how to ensure participation of the hard-to-reach. Can we apply learnings and amplify our successful experiences in building health security for these communities to other nations with similar demographic challenges?
- How can Canada build on the work of the National Advisory Committee on Immunization (NACI), and many provinces, which produced guidelines to prioritize vaccination for the most vulnerable during the pandemic, factoring in elements that included working and living conditions?
Recommendation 1.3 – Canada must become one of the best customers of global supply chains for essential goods and services by developing innovative procurement mechanisms and institutionalizing those developed during the pandemic.
Canada is only two percent of the global life sciences market, but that doesn’t leave the country without influence. Just as Canada must develop and produce global tradeables, it must be equally strategic about its role as a consumer in global health security supply chains.
For example, Health Canada has played a major international role in exploring the implications of the transfer of antimicrobial resistance from veterinary animals to humans, as well as assessing how the regulatory flexibility that produced speedy drug approvals in response to COVID could be adapted to any emerging future antimicrobial resistance threats.[16]
It is possible to create a comparative advantage around being the best customer by building on Canada’s first-class procurement reputation during the pandemic. Innovative procurement mechanisms could include using tools such as pre-purchase agreements, long-term contracts (perhaps modelled on the U.K. and France, where they have long-term contracts with companies to create domestic investment capabilities), and best-value-for-money rather than lowest-cost-compliant procurement systems.
Strategic questions:
- How will Canada maintain support from traditional suppliers during the next crisis?
- What contracting innovations can be designed to supplement Canada’s global image as a stable, peaceful democracy and positive reputation for investors?
- What mechanism would provide a clear market indicator of intent to purchase from particular vendors? How can we ensure there is market certainty for industries to invest in Canada, as well as a baseline level of capabilities to ramp up domestically during a crisis?
- How do federal and provincial governments pivot in the face of emerging risks and give the public service authority to make quick procurement decisions, providing fast, sufficient and guaranteed funding to implement those decisions?
- How does Canada develop a system that gathers post-market data about products such as vaccines and delivers real-time feedback to pharmaceutical manufacturers?
- How can an international appreciation be built around the strengths of the Canadian market, as well as our health-care systems, so our partners will want Canada within their supply chains?
- How should Canada respond to the proposal for a multinational pandemic treaty with a set of procurement rules that ensures global distribution of vaccines, diagnostics and therapeutics?[17]
Emergency management planning and preparedness
Recommendation 2.1 – Create standardized, anonymized electronic intelligence-gathering systems to collect and aggregate real-time data required for health emergency response decision-making.
Canada must close major gaps in its current health intelligence-gathering systems, including the lack of interoperability and real-time data exchange.[18] That starts with identifying the data that is essential to have before, during and after a health emergency. Having that real-time data at hand may also be a competitive advantage that Canada can use to develop its tradeables.
As things stand, even capturing a national picture of Canada’s overall health security during a crisis is a challenge. Manual data collected by provincial systems that operate too frequently in isolation risks incomplete and out-of-date reporting. As well, public health responses related to disease transmission/outbreaks, the efficacy of vaccines and their adverse effects, procurement, and health human resources management are spread across different ministries and jurisdictions — each with their own data system — within the federal and provincial governments.
The objective must be to build, perhaps by leveraging artificial intelligence, an accurate, real-time data set to determine the effectiveness of health emergency responses and enable decision-makers to quickly address gaps around issues such as rates of infection and vaccination, as well as monitoring the threat environment.
Strategic questions:
- Where can public health data infrastructure and intelligence-gathering systems be integrated to detect and plan for viral strains, outbreaks, health product availability and vaccine distribution
- How can data be better used to understand where products should be distributed once they have arrived in Canada, and who should receive them?
- How do we build data networks to provide national real-time inventory information on stockpiles of needed products to respond to health emergencies?
Recommendation 2.2 – Improve health emergency risk communications, integrating strategies to respond to misinformation and disinformation.
All risk communications[19] on behalf of government must acknowledge that comments and advice are based on the best available scientific information at the time of the statement. They must also note that science can evolve, and that subsequent evidence may change decisions, conclusions or advice.[20]
Inconsistent, and at times contradictory, communications on issues such as masking and the efficacy of specific vaccines led to public confusion during the pandemic, which opened the door to misinformation and contributed to the recent growth of polarization within Canadian society.[21]
Canada must create a system that ensures equity of communications across communities in multiple languages online, in print and broadcast across mainstream and social media. That includes information and outreach designed to meet the differing needs of remote, immigrant, Indigenous, urban and rural communities.
Strategic questions:
- How should Canada share timely, accurate information to counter the damage that may be caused by ignorant or malicious spreaders of misinformation and/or disinformation?
- How might Canada best engage with the newly established World Health Organization pandemic hub to share information and improve domestic preparedness for the next pandemic?[22]
Recommendation 2.3 – Conduct regular system-readiness testing through simulations and scenarios to probe for weaknesses in preparedness.
Canada must launch regular domestic health security tabletop exercises and crisis simulations involving the federal, provincial and territorial governments, industry, academics and experts to test health security and emergency management preparedness systems. This will complement Canada’s participation in similar WHO-organized exercises. The national simulations should include:
- Testing of communications and data systems on supply and demand for vaccines, medical devices, personal protective equipment, vials and therapeutics; and
- Data and reporting systems for managing and distributing stockpiled supplies.
Industry should also test its emergency response capabilities to address shortcomings. Such simulations should focus on validating or adjusting threat analyses and on replicating the pressure-cooker, time-scarce environment that was a constant constraint throughout the pandemic.
Strategic questions:
- Can Canada become a world leader in pandemic emergency response, a centre for best practices?
- Could Canada host an annual international congress on best practices and trends?
- The World Health Organization recently ran a pandemic crisis simulation with representatives from Global Affairs Canada in attendance. What was learned from that international exercise that can be applied domestically, either to run our own simulations or to identify where Canadian comparative advantages could become tradeables in global supply chains, helping make Canada a supplier of choice?
If not now, when?
This moment represents a unique opportunity to enhance the health security of Canadians for decades to come.
Industry, government and academia worked well together during the pandemic, accomplishing a huge amount in an extraordinarily short period of time. Trust among the various players remains, and there’s an opportunity to build on it. There’s a will to apply the lessons learned from COVID to be better prepared for whatever comes next — a will to win the peace.
PPF’s Life Sciences Forum is committed to supporting these efforts. By facilitating publicly interested dialogue and collaboration between the life sciences sector and government, the forum is helping to create an underpinning of trust necessary to launch investments and innovations that will shore up Canada’s health security. In partnership with the Government of Canada, and ideally aligned with a new Canadian health security institution, the forum’s members will help identify Canada’s economic and strategic advantages and tradeables that can be brought to international negotiations, thereby obtaining leverage to better respond to whatever health emergency may come next.
No one wants a repeat of the SARS experience, in which extensive post-mortem recommendations sat on the shelf with limited action taken,[23] leaving Canada to start almost from scratch when confronting COVID.
That must not happen again.
The public will never forget how the pandemic altered their lives and those of their families and friends, what worked to ease their worries and what didn’t. It is no surprise that Canadians now have little patience for delays. In the post-COVID world, it will simply not be good enough to fall back on yet more rounds of strategies and action plans that yield neither action nor change.
Pandemics are no longer historical events rooted in the distant past, or the domain of science fiction. Just as 100-year floods, fires and other climate disasters are now almost annual events, Canadians will experience a range of health threats in our lifetimes. All require detailed emergency preparedness planning, and medical countermeasures from Canada’s life sciences sector.
When the next health security emergency inevitably descends upon us, Canadians will expect an aggressive, strategic response, honed in the precious moments between crises. Let’s get to it.
- Canadian War Museum. (n.d.). Canada and the War; Canada and the War in Afghanistan. Veterans Affairs Canada. (n.d.); Korean War. Government of Canada. ↑
- Vaccine and Infectious Disease Organization. (2023). Next time we must do better: Building Canadian capacity for pandemic response, pp 5-8. ↑
- Marani, M., Katul, G.G., Pan, W.K., and Parolari, A.J. (2021). Intensity and frequency of extreme novel epidemics, 118(35). PNAS. doi:10.1073/PNAS.2105482118. ↑
- Carroll, L. (March 20, 2023). Deadly fungal infection spreading at an alarming rate, CDC says. CNBC. ↑
- Marani, M., Katul, G.G., Pan, W.K., and Parolari, A.J. (2021). Intensity and frequency of extreme novel epidemics, 118(35). PNAS. doi:10.1073/PNAS.2105482118. ↑
- Natural Resources Canada. (Aug. 23, 2023). National Wildfire Situation Report. Government of Canada. ↑
- Bahar, D. (Jan. 5, 2021). The secret sauce behind Israel’s successful COVID-19 vaccination program. Brookings Institution.↑
- Bingham, K., and Hames, T. (Dec. 5, 2023). The Long Shot: The Inside Story of the Race to Vaccinate Britain, p. xx. Oneworld Publications. ↑
- World Health Organization. (n.d.). Health Security. ↑
- Government of Canada. (June 23, 2023). COVID-19 vaccination: Vaccination coverage. ↑
- Government of Canada. (July 28, 2021). Canada’s Biomanufacturing and Life Sciences Strategy; Vaccine and Infectious Disease Organization. (2023). Next time we must do better: Building Canadian capacity for pandemic response, p. 19. ↑
- Auditor General of Canada. (Dec. 6, 2022). 2022 Reports 9 and 10 – COVID-19 vaccines. ↑
- Auditor General of Canada. (Nov. 10, 2021). Report 15 – Enforcement of quarantine and COVID-19 testing orders – Public Health Agency of Canada. ↑
- U.S. Government Accountability Office. (Feb. 11, 2021). Operation Warp Speed:Accelerated COVID-19 Vaccine Development Status and Efforts to Address Manufacturing Challenges. ↑
- Vaccine and Infectious Disease Organization. (2023). Next time we must do better: Building Canadian capacity for pandemic response, pp. 25-32. ↑
- International Coalitions of Medicines Regulatory Authorities. (November 2022). Antimicrobial Resistance Best Practices: Working Group Report and Case Studies. ↑
- Public Health Agency of Canada. (Aug. 24, 2023). Canada’s role in the development of an international pandemic instrument. Government of Canada. ↑
- Public Policy Forum. (January 2023). Taking Back Health Care: How to accelerate people-centred reform now, p. 11. ↑
- World Health Organization. (n.d.). Risk communication and community engagement. ↑
- Vaccine and Infectious Disease Organization. (2023). Next time we must do better: Building Canadian capacity for pandemic response, p. 21. ↑
- Ling, J. (Aug. 1, 2023). Far and Widening: The Rise of Polarization in Canada. Public Policy Forum. ↑
- World Health Organization. (n.d.). WHO Hub for Pandemic and Epidemic Intelligence. ↑
- Health Canada. (2003). Learning from SARS: Renewal of Public Health in Canada. Government of Canada; Vaccine and Infectious Disease Organization. (2023). Next time we must do better: Building Canadian capacity for pandemic response, pp.17-18. ↑
Acknowledgements
When the Public Policy Forum embarked on this report, we recognized that bolstering Canada’s health security would require the collective brainpower of creative, forward-thinking government leaders, as well as bright lights in the life sciences sector. And so it did. Insights generated by PPF’s Life Sciences Forum (LSF) contributed to the policy ideas and recommendations outlined in this report. We thank the following members of the LSF for their time, energy and willingness to share their experiences:
Supporting Partners:
- Astellas Pharma Canada, Inc.
- Stem Cell Network
- Moderna, Inc.
- Life Sciences Ontario
- Ipsen Biopharmaceuticals Canada, Inc.
- Canadian Agency for Drugs and Technologies in Health
- Life Sciences British Columbia
Strategic Partners:
- Health Canada
- Canadian Institute for Health Information
- adMare BioInnovations
- Hoffmann-La Roche Limited
- Roche Diagnostics
- Roche MedTech
- Johnson & Johnson Inc.
- Innovation, Science and Economic Development Canada
- Public Health Agency of Canada
- Bayer Inc.
- Eli Lilly Canada Inc.
Life Sciences Forum – Leadership Table:
- Mark Lievonen, Co-Chair, PPF Life Sciences Forum
-
Ilse Treurnicht, Co-Chair, PPF Life Sciences Forum
-
Simon Kennedy, Deputy Minister, Innovation, Science and Economic Development Canada
-
Ritu Banerjee, Assistant Deputy Minister
-
Innovation, Science and Economic Development Canada
-
Stephen Lucas, Deputy Minister, Health Canada
-
Eric Bélair, Associate Assistant Deputy Minister at Health Canada
-
Patrick Dicerni, Assistant Deputy Minister, Ministry of Health, Ontario
-
David O’Toole, President & CEO, Canadian Institute for Health Information (CIHI)
-
Lesia Babiak, Head, Canada Government Affairs & Policy, Johnson & Johnson
-
Gordon McCauley, President and CEO, adMare BioInnovations
-
Suzanne McGurn, President and CEO, CADTH
-
Cate Murray, President, Stem Cell Network
-
Ed Dybka, General Manager, Ipsen Biopharmaceuticals Canada
-
Patricia Gauthier, Country General Manager, Moderna
-
Stefan Raos, Interim Country General Manager, Moderna
-
Brigitte Nolet, President and CEO, Roche Canada Pharma
-
Jodi Butts, Senior Consultant, WATSON Advisors
-
Karen Oldfield, President & CEO, Nova Scotia Health Authority
-
Chad Mitchell, Assistant Deputy Minister, Pharmaceutical and Supplementary Benefits, Government of Alberta
-
Stephen Bent, Former Vice President, COVID-19 Vaccine Rollout Task Force, Public Health Agency of Canada
-
James Brodie, General Manager, Johnson & Johnson MedTech (JJMT)
-
Michele D’Elia, Executive Director, Medical & Scientific Affairs, Roche Diagnostics
-
Jill Daley, General Counsel, Eli Lilly Canada Inc.
-
Frank Stramaglia, General Manager, Astellas Pharma Canada
-
Berkeley Vincent, President, Janssen Inc.
-
John Wilkinson, Board Member, Life Sciences Ontario
-
Frank Béraud, Chief Executive Officer, Montréal InVivo
-
Edward Greenspon, President & CEO, Public Policy Forum
-
Kathleen Gnocato, Vice-President, Strategic Engagement, Public Policy Forum
-
Marian Campbell Jarvis, Senior Policy Advisor, Public Policy Forum
-
Pierre Sabourin, Fellow, Public Policy Forum
- Wendy Hurlburt, President and CEO, Life Sciences British Columbia